The largest barrier in conducting CBD research is perhaps the dubious legal classification of CBD. It was only through the 2018 Farm Bill that hemp-derived CBD became recategorized from a Schedule 1 drug, which previously limited the amount and type of research that can be done.
Besides this, there are still difficulties in gaining access to the quantity and quality of cannabis products needed for research.
Because of the challenges and barriers in conducting CBD research, including regulatory and supply barriers, there is still a lot we don’t know about how CBD can provide such a wide range of physical and psychological benefits. Perhaps the largest barrier is the still dubious legal classification of CBD.
Schedule 1 drugs are considered by the Food and Drug Administration to have no medical value and a high risk of abuse. Despite the many credible studies into the therapeutic use of these drugs, which include marijuana, magic mushrooms, and LSD, this classification limits the amount and type of research that can be done. While CBD doesn’t generate a high like its psychoactive cousin THC, it wasn’t until Congress’ passing of the 2018 Farm Bill was hemp-derived CBD recategorized from a Schedule 1 drug.
While the 2018 Farm Bill helps, there are still difficulties to gain access to the quantity and quality of cannabis products needed for research. For instance, all researchers must gain approval from several federal review boards before being allowed to research. Even then they aren’t guaranteed funding and they still must apply for licensing from the Drug Enforcement Agency, which may deny the license due to their role in prosecuting drug-related crimes.
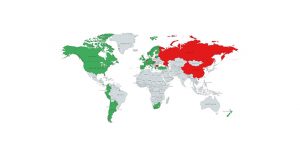
There is still some confusion regarding the legal status of CBD around the world despite the supplement’s increasing popularity. Most of this uncertainty revolves around...
Read More
While the current scientific consensus is there is no evidence pure CBD doesn’t cause widespread public harm, CBD products exist in another reality. As recently...
Read More
As hemp and marijuana are essentially the same plants, just with different levels of THC, CBD has existed in a bit of a legal gray...
Read More